Chromatography for Plant Pigments
Chromatography For Plant Pigments
How chromatography is used to study plant pigments?
Words ‘Plant pigments’ and ‘chromatography’ reminds us of the beginning of chromatographic science. A Russian Botanist ‘Mikhail Tsvet’ invented chromatography in 19th century during his research on separation of plant pigments.
Tsvet had managed to separate a mixture of plant pigments, including chlorophyll, on a column packed with finely ground calcium carbonate, using petroleum ether as the mobile phase.
Natural pigments such as chlorophylls, chlorophyll derivatives, xanthophylls, carotenoids, anthocyanins, betacyanin’s are abundant in plants or plant derived products.
Plant parts such as coloured flowers and fruits are because of carotenoids, flavonoids, anthocyanins or betalains. There are two main groups of betalains, the red-violet betacyanins and the yellow betaxanthins. Anthocyanins can be extracted by using solvent like methanol and often acid is added while doing extraction as most of the anthocyanins are pH sensitive.
Normal phase or reversed phase plates are used for separation of plant pigments depending on the nature of pigments intended to be separated. The flavonoid pigments are one of the numerous and most widespread groups of natural products.
The flavonoid class anthocyanins is the source of orange to blue colors in petals, fruits, leaves and roots. Other flavonoid contributes to yellow flower color either by cooccurring with carotenoid or by replacing them in about 15% of all plant species.
Until now various anthocyanin standards have been identified in different plant species e.g. pelargonidin, cyanidin, peonidin, delphinidin, petunidin and malvidin etc. Different solvent systems are used depending on type of stationary phase to be used.
For normal phase plates solvent system like n butanol: acetic acid: water 40:10:20 v/v/v, ethyl acetate: isopropanol: water 65:25:10 v/v/v, Ethyl acetate: toluene: water: formic acid 10:3:0.8:1.1 v/v/v/v. For reverse phase solvent systems like methanol: water: formic acid 40:60:2 v/v/v can be used.
Let’s take one example to understand how HPTLC can be used for separation of plant pigments (In this case flavonoids) for the separation and to see differentiation between yellow and red Butea flowers:
Comparative HPTLC fingerprint of Butea monosperma (Yellow and Red flowers):


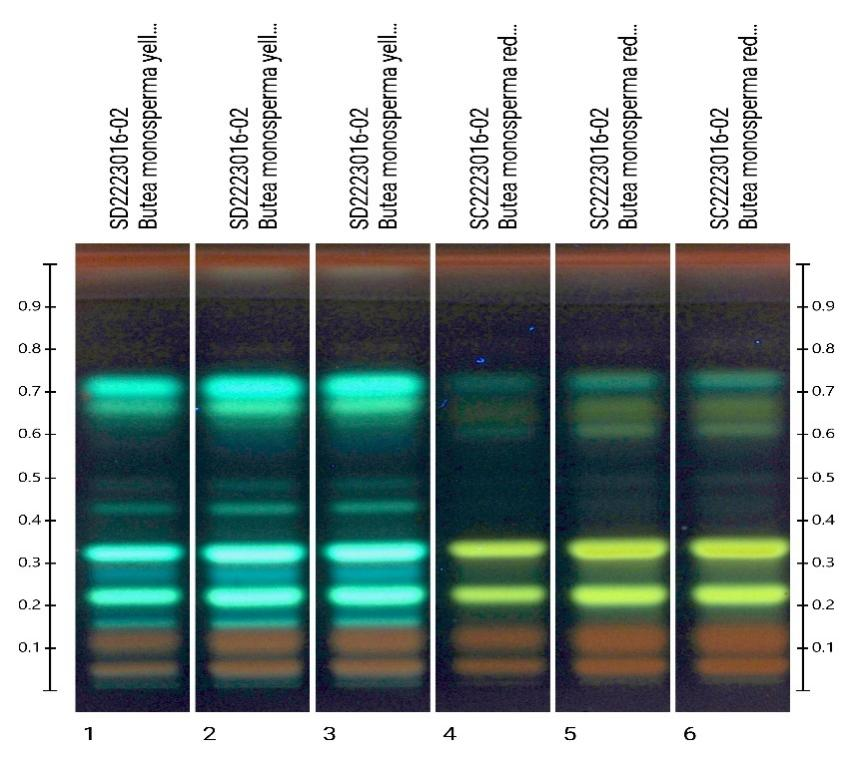
HPTLC method was developed for differentiation between Butea monosperma yellow flowers and Butea monosperma red flowers.
After development plate was derivatized with Natural product and anisaldehyde sulphuric acid reagent and image was taken at 366nm. Yellow fluorescent bands were observed at Rf 0.23, 0.34 in Red butea flowers which differentiates red from yellow butea flowers.
Whereas, aqua green fluorescent bands were observed at Rf 0.17, 0.23, 0.34 and 0.43 in yellow butea flowers only. These bands can be used for differentiation of yellow and red Butea flower samples.
Reference: Practical Thin Layer Chromatography-A Multidisciplinary Approach, B. Fried & J. Sherma, CRC Press, Taylor and Francis Group, 2017.
Anchrom Enterprises Pvt. Ltd is one of the leaders in HPTLC in food analysis. Please contact us at lab@anchrom.in for HPTLC analysis of plant extracts, drugs, ingredients in cosmetics, and forensic science.

Comments
Post a Comment